The 340B Drug Pricing Program is a US federal government program that requires drug manufacturers to provide outpatient drugs to eligible health care organizations and covered entities at significantly reduced prices. The program was created in 1992 as an extension of the Medicaid Drug Rebate Program (MDRP) to lower the cost of pharmaceuticals reimbursed by state Medicaid agencies. The MDRP requires drug companies to enter into a rebate agreement with the Secretary of the Department of Health and Human Services (HHS) as a precondition for coverage of their drugs by Medicaid and Medicare Part B. Under the 340B program, pharmaceutical manufacturers must enter into a pharmaceutical pricing agreement (PPA) with the HHS Secretary, agreeing to provide discounts on covered outpatient drugs purchased by specified providers, known as covered entities, that serve the nation's most vulnerable patient populations. The purpose of the 340B program is to enable covered entities to stretch limited federal resources, reach more eligible patients, and provide more comprehensive services.
| Characteristics | Values |
|---|---|
| Year of creation | 1992 |
| Administering body | Office of Pharmacy Affairs (OPA) |
| Administering body location | Rockville, MD |
| Administering body contact number | 1-888-340-2787 |
| Administering body email | [email protected] |
| Purpose | To enable covered entities to "stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services" |
| Who is eligible to participate in the program | Six categories of hospitals and ten categories of non-hospital covered entities |
| Categories of hospitals | Disproportionate share hospitals (DSHs), children's hospitals and cancer hospitals exempt from the Medicare prospective payment system, sole community hospitals, rural referral centers, and critical access hospitals |
| Categories of non-hospital covered entities | Federally qualified health centers (FQHCs); FQHC “look-alikes”; state-operated AIDS drug assistance programs; the Ryan White Comprehensive AIDS Resources Emergency (CARE) Act clinics and programs; tuberculosis clinics; black lung clinics; Title X family planning clinics; sexually transmitted disease clinics; hemophilia treatment centers; Urban Indian clinics; and Native Hawaiian health centers |
| Compliance monitoring and enforcement | Conducted by the Health Resources and Services Administration (HRSA) |
What You'll Learn
- The 340B Drug Pricing Program was created in 1992 to reduce drug prices for eligible healthcare organisations and covered entities
- The program is administered by the Office of Pharmacy Affairs (OPA) and requires manufacturers to enter into a pharmaceutical pricing agreement (PPA)
- Covered entities include six categories of hospitals and ten categories of non-hospital covered entities
- The 340B program prohibits the resale or transfer of discounted outpatient drugs to anyone other than a patient of the covered entity
- Compliance with the 340B program is monitored and enforced through audits, recertification, and penalties for violations

The 340B Drug Pricing Program was created in 1992 to reduce drug prices for eligible healthcare organisations and covered entities
The 340B Drug Pricing Program is a US federal government program created in 1992 to reduce drug prices for eligible healthcare organisations and covered entities. The program requires drug manufacturers to provide outpatient drugs at significantly reduced prices to eligible healthcare organisations and covered entities.
The program was created to address the issue of rising drug costs, which was particularly impacting safety-net providers, i.e., healthcare organisations that serve a large number of low-income and uninsured patients. By enacting the 340B program, Congress aimed to provide similar relief to safety-net providers as it had provided to the Medicaid program with the Medicaid rebate law in 1990.
Under the 340B program, eligible healthcare organisations and covered entities can achieve average savings of 25% to 50% on pharmaceutical purchases. This allows them to stretch their limited resources, reduce the price of outpatient pharmaceuticals for patients, and expand health services to the patients and communities they serve. For example, hospitals use 340B savings to provide free care for uninsured patients, offer free vaccines, and provide services in mental health clinics.
To be eligible for the 340B program, healthcare organisations must be defined as "covered entities" under federal law. Covered entities include six categories of hospitals: disproportionate share hospitals (DSHs), children's hospitals, rural referral centres, sole community hospitals, and critical access hospitals. In addition, there are ten categories of non-hospital covered entities that are eligible based on receiving federal funding, such as federally qualified health centres and state-operated AIDS drug assistance programs.
To participate in the 340B program, eligible organisations must register, enrol, and comply with all program requirements. They must also recertify their eligibility annually and participate in audits conducted by the Health Resources and Services Administration (HRSA) and drug manufacturers.
The 340B program has been the subject of controversy and legal challenges, with drug manufacturers expressing concerns about its fast expansion and the lack of transparency and oversight. However, policymakers and government agencies largely support the program, recognising its importance in helping safety-net providers care for vulnerable patient populations.
Exploring the Economics of Term Insurance: Unraveling the Affordability Factor
You may want to see also

The program is administered by the Office of Pharmacy Affairs (OPA) and requires manufacturers to enter into a pharmaceutical pricing agreement (PPA)
The 340B Drug Pricing Program is administered by the Office of Pharmacy Affairs (OPA), which is located within the Health Resources and Services Administration (HRSA) within the Department of Health and Human Services (HHS). The OPA is responsible for designing and implementing policies and procedures to enforce agency objectives and assess program risk.
The program requires pharmaceutical manufacturers participating in Medicaid to enter into a pharmaceutical pricing agreement (PPA) with the Secretary of Health and Human Services (HHS). Under the PPA, manufacturers agree to charge a price for covered outpatient drugs that will not exceed an amount determined under the statute (340B ceiling price). The 340B ceiling price is the maximum price that covered entities may pay for drugs, and manufacturers are required to offer these drugs to covered entities at or below this price.
The PPA must be signed by manufacturers as a condition for participating in the Medicaid program. It is important to note that signing the PPA does not prohibit manufacturers from charging a lower price for covered outpatient drugs. Additionally, manufacturers cannot condition the offer of 340B discounts on a covered entity's assurance of compliance with 340B Program requirements.
The OPA has developed an integrated 340B Office of Pharmacy Affairs Information System (340B OPAIS) to facilitate the registration and management of covered entity and manufacturer information. The 340B OPAIS includes a secure web-based application that allows manufacturers to upload and submit their quarterly pricing data. HRSA uses this data to validate manufacturer-submitted information, ensuring compliance with the program's requirements.
To participate in the 340B program, manufacturers must meet several requirements, including signing the Pharmaceutical Pricing Agreement and Addendum, registering with 340B OPAIS, submitting quarterly pricing data, and complying with all 340B Program requirements. By adhering to these guidelines, manufacturers play a crucial role in ensuring the successful administration of the program by the OPA.
Understanding Bill Insurance: Protecting Your Finances from Unforeseen Costs
You may want to see also

Covered entities include six categories of hospitals and ten categories of non-hospital covered entities
The 340B Drug Pricing Program is a US federal government program that requires drug manufacturers to provide outpatient drugs at significantly reduced prices to eligible healthcare organizations and covered entities. Covered entities include six categories of hospitals and ten categories of non-hospital covered entities.
The six categories of hospitals that are eligible to participate in the program are:
- Disproportionate Share Hospitals (DSHs)
- Children's Hospitals and Cancer Hospitals exempt from the Medicare prospective payment system
- Sole Community Hospitals
- Rural Referral Centers
- Critical Access Hospitals (CAHs)
To be eligible, hospitals in each of the categories must meet the following requirements:
- They must be owned or operated by a state or local government, a public or private non-profit corporation with governmental powers granted by a state or local government, or a private non-profit hospital with a contract with a state or local government to provide care to low-income individuals who are not eligible for Medicare or Medicaid.
- They must have a "disproportionate share hospital (DSH) adjustment percentage" above a certain level.
The ten categories of non-hospital covered entities that are eligible based on receiving federal funding are:
- Federally Qualified Health Centers (FQHCs)
- FQHC "look-alikes"
- Ryan White HIV/AIDS Program Grantees
- Tuberculosis, Black Lung, Family Planning, and Sexually Transmitted Disease Clinics
- Hemophilia Treatment Centers
- Public Housing Primary Care Clinics
- Homeless Clinics
- Urban Indian Clinics
- Native Hawaiian Health Centers
- State-operated AIDS Drug Assistance Programs
Covered entities that participate in the 340B program can purchase outpatient drugs at significantly discounted prices, which helps them stretch limited federal resources, reduce costs for patients, and expand health services for their communities.
Unraveling the Nuances of Billing Secondary Insurance: A Comprehensive Guide
You may want to see also

The 340B program prohibits the resale or transfer of discounted outpatient drugs to anyone other than a patient of the covered entity
The 340B Drug Pricing Program is a US federal government program that requires drug manufacturers to provide outpatient drugs at significantly reduced prices to eligible health care organizations and covered entities. The program was created in 1992 to allow covered entities to "stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services."
The 340B law prohibits the resale or transfer of discounted outpatient drugs to anyone other than a patient of the covered entity. This is commonly referred to as "diversion." The Health Resources and Services Administration HRSA has defined a covered entity patient through a Federal Register notice published on October 24, 1996.
To be considered a patient of a covered entity, an individual must meet the following criteria:
- The covered entity must have an established relationship with the individual, defined as maintaining health records of the individual.
- The individual must receive a health care service for which the covered entity is responsible.
- The individual must receive a health care service from the covered entity consistent with qualifying grant funding or "look-alike status". Hospitals are exempt from this requirement.
Covered entities are allowed to dispense discounted medication to both uninsured patients and patients covered by Medicare or private insurance. When a covered entity treats an insured patient with discounted medication, the federal government or the patient's private insurance reimburses the entity for the full price of the medication. The entity can then retain the difference between the reduced price it pays for the drug and the full amount for which it is reimbursed.
The 340B program has been the subject of ongoing controversy due to limited program oversight and transparency in how covered entities use funds generated by the program. Despite this, the program has provided financial help to hospitals serving vulnerable communities by allowing them to manage rising prescription drug costs.
American Family Insurance: Understanding Their Billing Practices and What Lies Ahead
You may want to see also

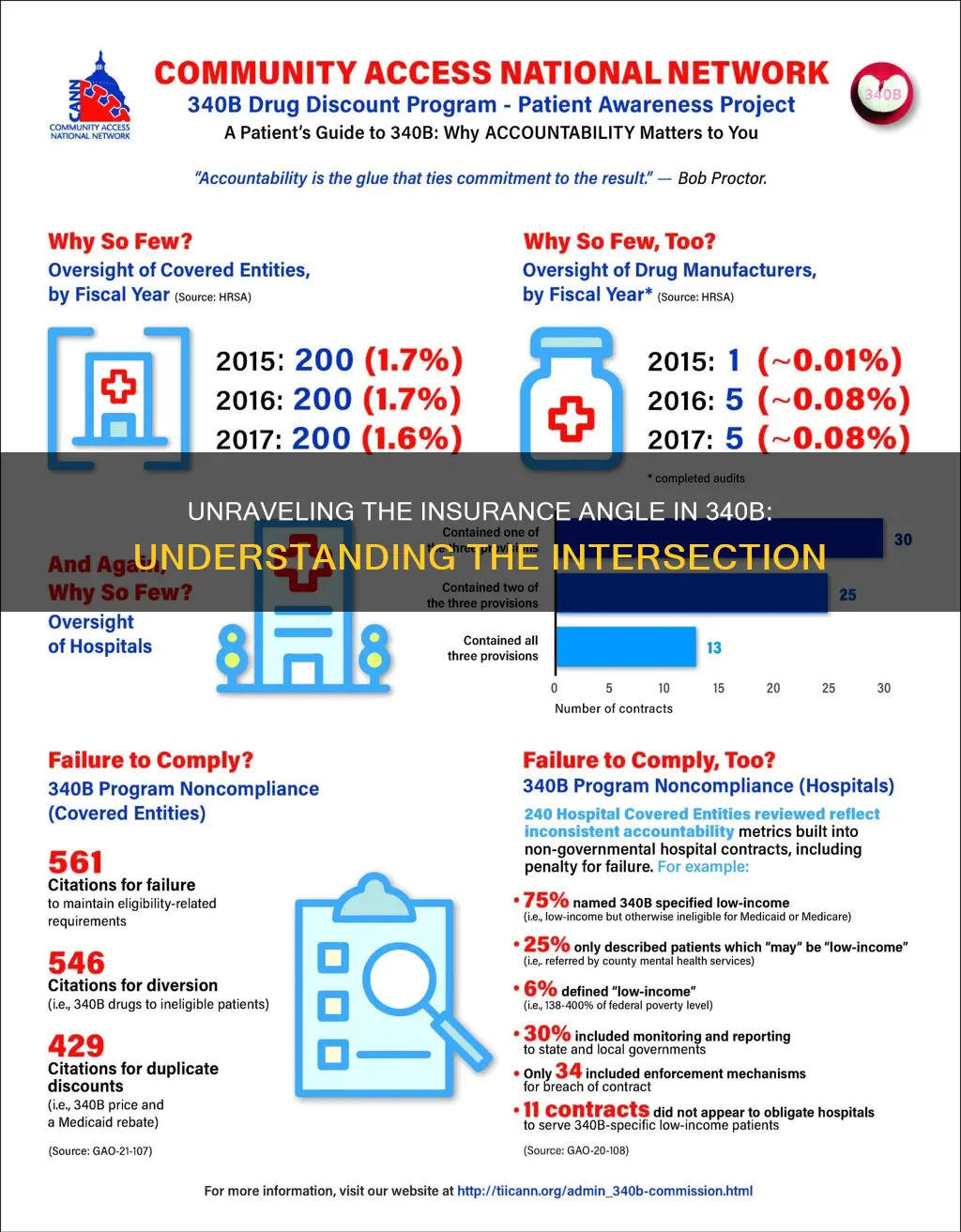
Compliance with the 340B program is monitored and enforced through audits, recertification, and penalties for violations
Audits
The Health Resources and Services Administration (HRSA) is responsible for conducting audits of covered entities and manufacturers to enforce requirements for these stakeholders. HRSA has the authority to audit covered entities for compliance with 340B Drug Pricing Program requirements. Covered entities are also subject to audit by the manufacturer or the federal government. Manufacturers are subject to auditing by HRSA to ensure compliance with the 340B Program.
Recertification
Annual recertification gives covered entities an opportunity to review their 340B Drug Pricing Program responsibilities and re-attest to being currently in full compliance. All 340B entities are required to recertify each year. The authorizing officials at 340B covered entities must attest to 340B compliance during recertification, including compliance at contract pharmacies.
Penalties for Violations
Covered entities and manufacturers are subject to penalties if they violate 340B program requirements. For covered entities, the penalty for failing to comply with the program’s diversion and duplicate discount provisions is repayment of the discounts back to the manufacturer. Where a diversion violation is knowing and intentional, covered entities may be required to pay interest on the discounts that they refund. If the diversion violation is systematic and egregious as well as knowing and intentional, a covered entity may be disqualified from 340B participation for a reasonable time, to be determined by HRSA. For manufacturers, the consequence for charging in excess of the ceiling price is to refund those overcharges. HHS is also authorized by statute to impose civil monetary penalties on companies that knowingly and intentionally overcharge covered entities for 340B drugs. The only other penalty for manufacturer non-compliance is termination of the manufacturer’s pharmaceutical pricing agreement which, in turn, would mean that the manufacturer’s covered outpatient drugs are excluded from Medicaid and Medicare Part B coverage.
Juggling Dual Coverage: Navigating Billing for Two Insurances
You may want to see also
Frequently asked questions
The 340B Drug Pricing Program is a US federal government program created in 1992 that requires drug manufacturers to provide outpatient drugs to eligible health care organizations and covered entities at significantly reduced prices.
The purpose of the 340B Drug Pricing Program is to allow covered entities to "stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services."
Facilities that believe they meet the criteria of a "covered entity" can apply to participate in 340B by completing the online registration process during the first two weeks of any calendar quarter. Once admitted into the program, covered entities are entitled to receive discounts on all eligible covered outpatient drugs.







